Kid Definition Ionic Bond. A glycosidic bond forms by a condensation reaction which means that one water molecule is produced during formation of a glycoside. An ionic bond is the electrostatic forces of attraction between a non metal and a metal ion in a giant ionic crystal lattice this occurs when charged atoms attract this happens after a metal atom loses one or more of its electrons to the non metal atom.
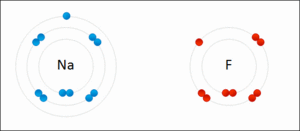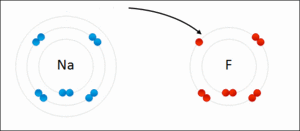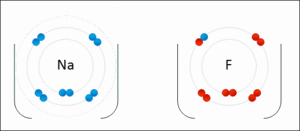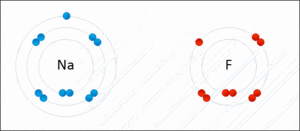
They generally occur between a metallic and a non metallic atom. Such a bond forms when the valence outermost electrons of one atom are transferred permanently to another atom. Ionic bond type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.
Covalent bonds have a definite shape while ionic bonds do not have a definite shape.
There are two types of bonds. Such a bond forms when the valence outermost electrons of one atom are transferred permanently to another atom. Kids net au dictionary definition. The greater the difference in charge between the metal and non metal ion the stronger the ionic bond.